How to Implement an ML/AI-Based Patient Finding Strategy and Improve Outcomes
Industry education for life science teams at any experience level
Introduction: Overcoming Patient Finding Challenges with Augmented Intelligence
Healthcare and life sciences companies need a better, more informed approach to critical decision-making throughout the product lifecycle, especially for rare and specialty diseases. The current industry standard relies on assumptions about patient populations, which creates inefficiencies. For instance, without understanding true patient demand, an accurate market assessment (which is needed to confirm that drug research, development and commercialization efforts are warranted) is impossible. Likewise, with no knowledge of where patients reside, designing a viable clinical recruitment strategy is unlikely — protocols may go through several revisions as trials drag on – all at a high cost. It’s also more difficult to maintain commercial effectiveness if insight into the undiagnosed patient population and their treating physicians is missing.
Instead of considering those who are undiagnosed, most companies only focus on known patients, which informs a guess about the total or future patient population. While this may be good enough for high-prevalence diseases such as diabetes, it falls short for rare and specialty conditions. Traditional vendors are also unable to incorporate real time results, rendering outputs obsolete as soon as they are delivered. These projects are created on a one-time basis, are generally “off-the-shelf” and highly dependent on internal resources (which creates a lag) – as well as expensive.
At IPM.ai, we take a dynamic precision-based approach to patient-finding. Using ML/AI-driven augmented intelligence, powered by real world data refreshed weekly, we enable brands to uncover and activate anonymized patients and their HCPs at key intervals of the product lifecycle. Not only does this lead to better outcomes for all parties from the start, but it also creates cost-saving opportunities. By relying on highly advanced ML/AI, humans can better strategize and optimize decision-making.
Most rare and specialty disease patients are misdiagnosed or undiagnosed, and therefore unaware of their exact condition. In fact, ninety-five percent of rare and specialty diseases have no pharmacological treatment options, which is devastating for patients and their families. This is often because diffuse symptomatology that affects multiple systems and presents differently in each patient complicates diagnosis. As specialty doctors are experts in one area, such as the heart or kidney, they often don’t recognize rare diseases that often have complex symptomatology. This can lead patients down a “diagnostic odyssey” in which they are incorrectly diagnosed by different specialists for years and continuously prescribed the wrong medications. These useless medicines can cause side effects that are then confused with internal symptoms, further obscuring the underlying health issue and adding another challenge to diagnosis.
Some rare and specialty diseases are so niche that they completely lack a diagnosis code – so even if an HCP can identify a condition, they aren’t able to register it. This fragments care further and is at least partially to blame for a lack of rare disease therapies in development.
Low prevalence rates, exacerbated by missing ICD-10 classification codes essentially render patients invisible, forcing them to suffer in silence. Life sciences companies that want to address this crucial market face massive R&D challenges and logistical hurdles. For instance, they are often unable to gauge a true patient population since undiagnosed and misdiagnosed patients are not factored in – making it difficult to obtain funding to start working on a drug. Likewise, they can’t easily reach the patients needed to test a therapy in clinical trials, further delaying the path to approval.
However, by relying on real world health data derived from claims data and machine learning/artificial intelligence (ML/AI) it’s possible to create models that reveal the ideal de-identified patient and their healthcare ecosystem, regardless of their current diagnosis status. This technology helps prioritize which HCPs to engage so they can be intercepted at key points of the patient treatment journey, improving health outcomes while benefiting the life sciences brand.
Technology has turned the promise of precision medicine into a reality; it’s time to stop relying on outdated approaches to rare disease patient-finding. Instead, companies must innovate and employ an ML/AI powered system of insight to transform how rare and specialty disease patients are diagnosed – and improve lives in the process.The clinical promise of precision medicine is an analytical reality
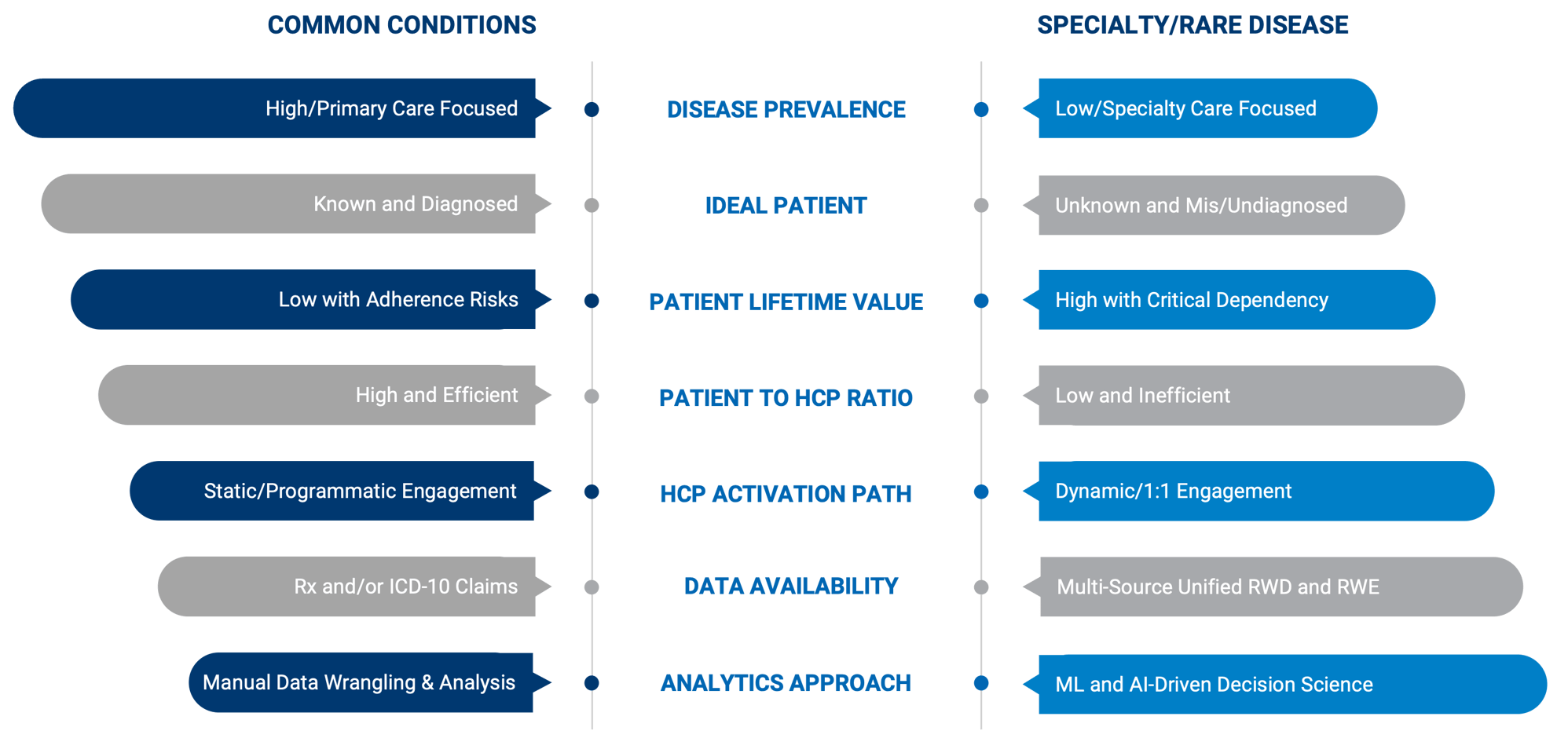
WHAT ARE THE DIFFERENCES BETWEEN RARE DISEASES AND COMMON CONDITIONS?
Common conditions are easy to diagnose, affect many and have available treatments, whereas rare and specialty diseases are defined by niche patient populations that are often undiagnosed or misdiagnosed and have no viable therapies.
HOW ARE RARE AND SPECIALTY DISEASE PATIENTS TRADITIONALLY TARGETED?
Rare and specialty disease patients are typically targeted through patient registries or advocacy groups. Companies may also mine lab and genetic data or turn to Key Opinion Leaders (KOLs) for insight. However, these approaches typically fall short as they exclude the majority of the patient population that remain undiagnosed or misdiagnosed.
WHY ARE CONVENTIONAL PATIENT FINDING APPROACHES MOSTLY INEFFECTIVE FOR RARE AND SPECIALTY DISEASE PATIENTS?
Conventional patient finding approaches are ineffective and fall short for rare and specialty disease patients because the conditions are largely unknown and difficult to recognize. Only 500 of these 7,000 rare and specialty diseases have been formally classified through an ICD-10 code and prevalence is typically less than 5 out of every 10,000 people. Low incidence rates render patient finding via programmatic advertising, social media outreach and search engine optimization wasteful. Although turning to patient registries, advocacy groups, mining lab and genetic data or turning to Key Opinion Leaders (KOLs) may uncover an initial cluster of patients, it is not scalable.
The fragmented nature of rare diseases negatively affects drug development. For instance, approximately 81% of clinical trial participants for rare and specialty drugs are routinely deemed medically-ineligible – meaning companies are unable to connect with the right patients in the testing phases. With a 131-month average cycle time for a phase I-III rare disease investigation (68% longer than a common disease), costs exceeding $100 million for a phase III study, and six times the number of sites required for enrollment compared to those for non-rare conditions, it’s no wonder that 90% of these studies are suspended or outright terminated. The impact is profound as it perpetuates a lack of treatment options.
Creating a Real Challenge for Life Science Companies
0 mos
$0 M
0%
0X
0%
$0 M
Creating a Real Challenge for Life Science Companies
131mos
the average cycle time for Phase I-III rare disease investigations -68% longer than conventional drugs
$103M
the average direct costs to conduct a Phase III trial of an investigational rare disease treatment
90%
of clinical investigations for rare diseases fail to meet patient recruitment goals
6x
the number of sites required to recruit 1/4 the number of patients, compared with those for non-rare diseases
81%
of patients screened for inclusion in a rare disease clinical trial are deemed ineligible to enroll
$2M
the average cost per day when a rare disease study is delayed due to lack of enrollment
The inability to diagnose rare and specialty patients places a tremendous burden on these individuals and their families as well as the healthcare system overall, which is further strained by the cost of a misdiagnosis.
WHAT IS THE COST OF RARE AND SPECIALTY DISEASES?
Rare conditions cost the United States more than $1 trillion annually. According to a study by EverydayLife/Lewin the economic burden of rare disease on patients is $449 billion in direct medical spend, $437 billion in indirect costs and $111 billion in non-medical costs.
HOW LONG IS A TYPICAL RARE DISEASE PATIENT JOURNEY?
A typical rare disease patient journey spans 7.6 years from initial onset to correct diagnosis, though it can take over a decade. Of the approximately 30 million Americans with rare diseases, patients will see an average of seven different HCPs before their condition is identified.
WHAT IS A DIAGNOSTIC ODYSSEY?
Rare and specialty disease patients face a difficult and unpredictable healthcare journey, which is described as a diagnostic odyssey. This phrasing is indicative of the patient experience because it involves so many steps, from being shuffled between doctors to taking unnecessary tests and medication, and almost inevitably receiving a misdiagnosis and subsequently being treated for the wrong condition.
WHAT IS AI?
Artificial intelligence (AI) involves the algorithms and processes that mimic cognitive human function to simulate intelligence.
WHAT IS ML?
Machine learning (ML) is a branch of AI that focuses on teaching machines to automatically improve and develop through supervised, unsupervised, or reinforcement learning algorithms.
WHAT IS RWD?
Real world data is claims data derived from health events such as visiting a doctor or filling a prescription. This is coupled with social determinants of health data that indicate lifestyle characteristics, gathered through spending history. RWD creates a more robust and accurate patient profile than data collected from online sources, for example.
Health Claims
Integrated Affiliations
Social Determinants of Health
Clinical Stages
Health Claims
- 300M+ Unique Patient Journeys
- Over a decade of history with weekly updates across TAs
- 1st Party Input Data lab, trial, EHR, hub and more
Integrated Affiliations
- National HCO Coverage across 100% of hospitals, IDNs, systems and many more
- 95% Accuracy on account-level insights
- Referral Mapping network and affiliation patterns between providers and accounts
Social Determinants of Health
- 80% US Households
- 3,700+ consumer attributes
- 65 billion consumer records
Clinical Stages
- Integrated Global R&D Intelligence
- Visibility to Historical and Active Trial Studies
- Principal Investigators mapped to NPI identifier
Health Claims
Health Claims
- 300M+ Unique Patient Journeys
- Over a decade of history with weekly updates across TAs
- 1st Party Input Data lab, trial, EHR, hub and more
Integrated Affiliations
Integrated Affiliations
- National HCO Coverage across 100% of hospitals, IDNs, systems and many more
- 95% Accuracy on account-level insights
- Referral Mapping network and affiliation patterns between providers and accounts
Social Determinants of Health
Social Determinants of Health
- 80% US Households
- 3,700+ consumer attributes
- 65 billion consumer records
Clinical Stages
Clinical Stages
- Integrated Global R&D Intelligence
- Visibility to Historical and Active Trial Studies
- Principal Investigators mapped to NPI identifier
WHAT IS THE IDEAL PATIENT POPULATION?
The ideal patient population (IPP) has a shared condition and health trajectory, and is medically eligible to receive a designated drug.
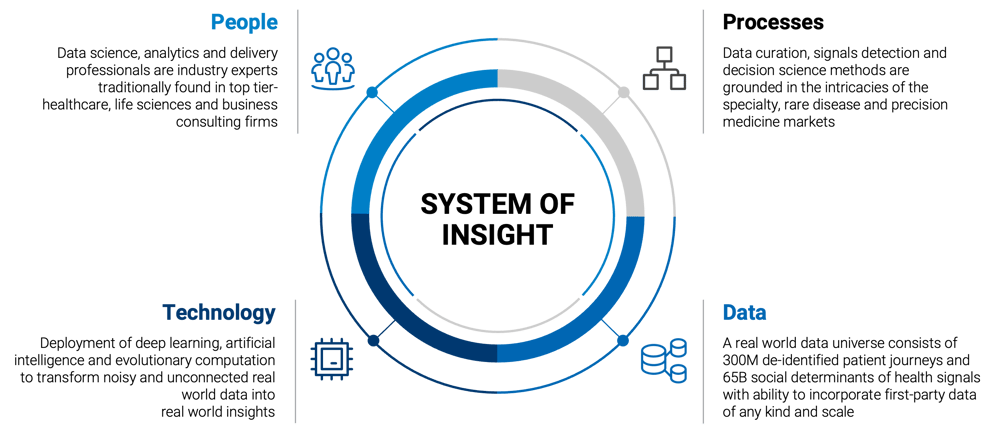
WHAT IS AUGMENTED INTELLIGENCE?
Augmented intelligence is a concept that utilizes ML/AI in conjunction with a curated pool of smart, real-world data to enhance, rather than replace, human decision-making.
WHAT IS RWD?
Real world data is claims data derived from health events such as visiting a doctor or filling a prescription. This is coupled with social determinants of health data that indicate lifestyle characteristics, gathered through spending history. RWD creates a more robust and accurate patient profile than data collected from online sources, for example.
HOW DOES IPM.AI USE ML/AI TO UNCOVER PATIENTS?
ML/AI enables healthcare and life sciences companies to uncover an ideal patient population and their healthcare ecosystem, accelerating the development and commercialization of specialty and rare disease therapies. To uncover hidden patients, IPM.ai creates a brand-specific ideal patient definition using privacy-safe real world data. Our extensive data universe consists of 300 million de-identified patient journeys going back over a decade, in conjunction with 65 billion anonymous social determinants of health signals and first-party sources of any type and scale such as genetic testing, laboratory research and epidemiological assessments. This broader patient universe is then scored based on their similarity to the original ideal patient population. ML then analyzes and learns from the differences in claims trajectories between the test and control group, ultimately predicting the right patients.
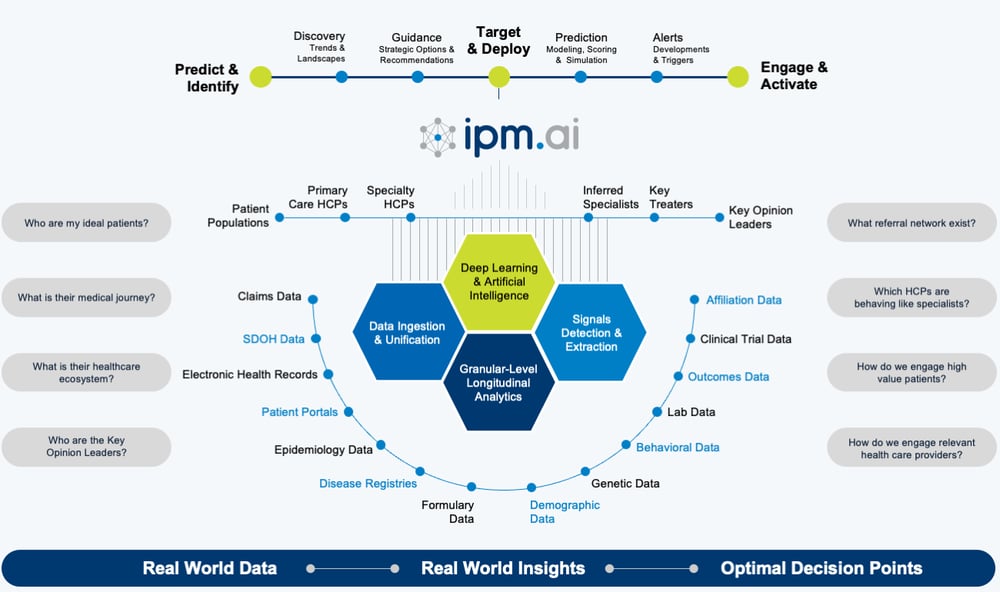
These anonymous patients are linked to identifiable physicians, based on recency of visit, frequency of claims, or to a triggering physician focused on a specific claim. The result is a list of HCPs and their corresponding patients that can be delivered directly to field teams for activation. Even after an initial list is completed, our clients continue to receive alerts as new patients are uncovered – and as our data refreshes weekly, this happens in near real time.
The ML/AI approach to patient finding can be applied across the product lifecycle, from rapidly assessing the size of a market for an orphan drug to uncovering patients with the highest propensity for successful clinical study screening, and those most likely to begin a newly launched or in-market therapy.
UNCOVERING THE IDEAL PATIENT AND THEIR HEALTHCARE ECOSYSTEM
WHAT ARE THE RESULTS OF USING ML/AI FOR PATIENT FINDING?
By relying on ML/AI in patient finding, IPM.ai has helped 80+ companies more effectively develop new molecules, conduct clinical studies, launch new drugs and accelerate commercial outcomes. Recent results include predicting metastasis progression at a 92% accuracy rate from early-stage diagnosis, uncovering 11,000 high value treaters for a neuromuscular condition – including those who were previously overlooked, lifting total patient volume by 60%, and uncovering 200,000 patients highly likely to have an under-diagnosed autoimmune disorder.
We have also enabled a rare disease client to project the existence of ~800 to 2,400 undiagnosed patients from only 100 documented cases. IPM.ai has helped a client with a therapy for an ultra-rare life-threatening disease (<6,500 patients in the US) increase the diagnosis rate by 40% and find ~10,000 patients who would benefit from a first-in-class therapy for a rare condition along with ~4,000 HCPs treating these likely patients. We also seamlessly delivered an average of 20 high-priority targets for a rare hematology treatment per sales rep, bi-weekly.
By focusing on patient finding for specialty and rare conditions defined by misdiagnosis or no diagnosis, we recently earned a recognition by Fast Company as the second most innovative organization on their prestigious Top 10 List of Most Innovative Data Science Companies.
Typical Results
0+
0%
0
0
0%
0
Typical Results
24+
IPM.ai projected the existence of ~800 to 2,400 undiagnosed patients for a client treating a rare disease with only 100 documented cases
40%
IPM.ai helped a client with a therapy for an ultra-rare life-threatening disease (<6,500 patients in the US) increase the diagnosis rate by 40%
10,000
IPM.ai found ~10,000 patients who would benefit from a client's first-in-class therapy for a rare condition and ~4,000 HCPs treating likely patients
400
IPM.ai found ~400 patients for a client's treatment of a rare muscular disorder that lacked an ICD-10 classification for effective diagnosis
60%
IPM.ai delivered a database with 13,000 top-decile HCPs accounting for 60% of total patient volume to a client with a first-in-market treatment
7,000
IPM.ai found ~7,000 HCPs treating hidden patients for a disease with less than 2,000 patients, raising the positive-patient ID rate
HOW ARE PATIENT IDENTITIES PROTECTED?
Our partner, Datavant, creates irreversible, site-specific tokens for each record in a two-step approach that allows common tokens to match records at the de-identified level without personal, identifiable information or protected health information ever entering the IPM.ai system. IPM.ai’s patented privacy-safe architecture and data processing methodology is HIPAA certified, uniquely positioning us to combine disparate data sets – including clients’ first-party data – without the need for a lengthy and expensive recertification process. IPM.ai can discover health care providers that treat ideal patients via our claims dataset with all identities kept completely anonymous.
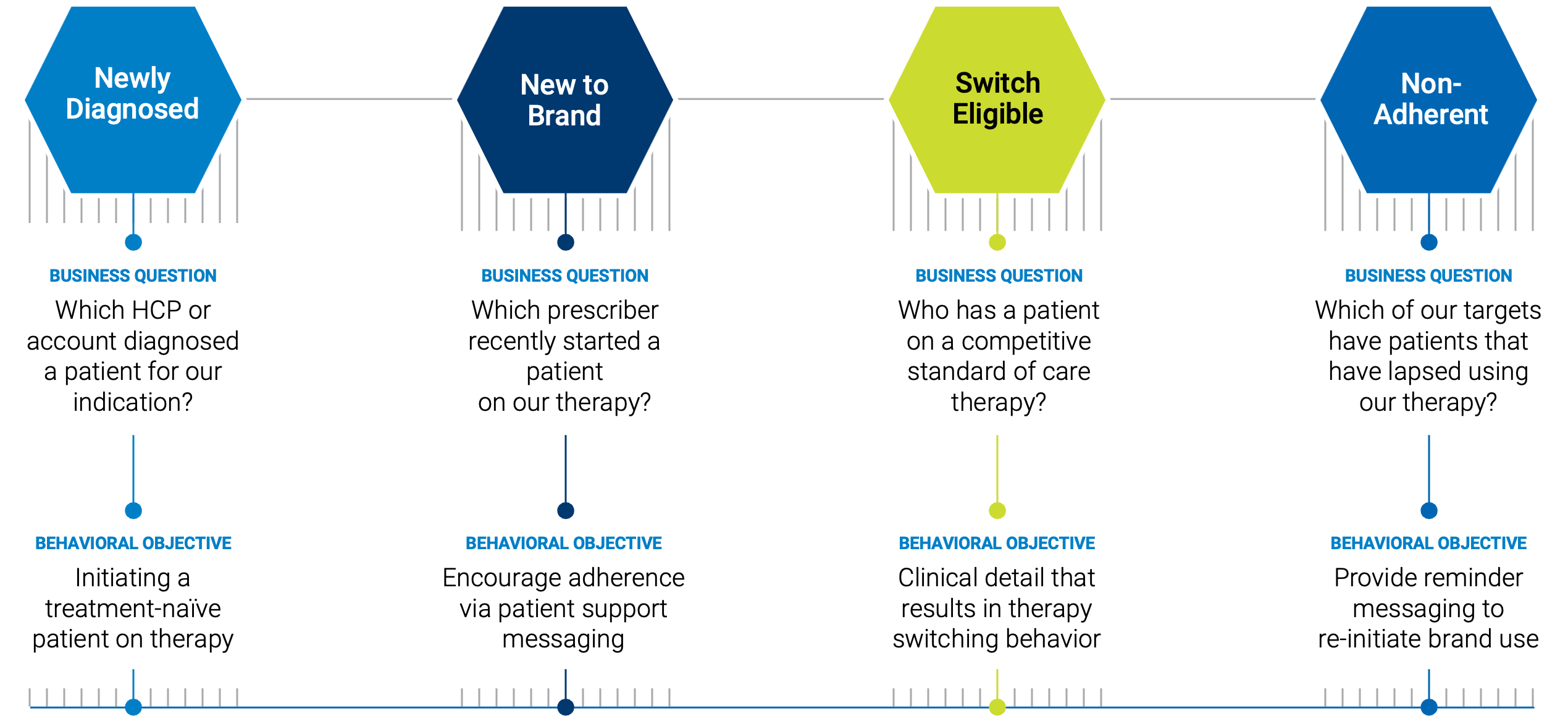
HOW CAN MY FIELD TEAMS BE ALERTED TO RARE AND SPECIALTY PATIENTS AS HEALTH EVENTS OCCUR?
Health events will trigger an alert in Veeva or the CRM of your choice. This alert will enable field teams to see the treating physician associated with the patient of interest. They can then outreach directly to the HCP and provide education on a drug, while informing them that there is a rare disease patient that can benefit from the therapy under their care. Each rep can be strategically deployed into the field at the precise moments in a patient’s treatment journey when a therapy or clinical trial has the greatest potential to result in a positive outcome.
IPM.ai’s weekly alerts are delivered directly to our clients, allowing them to take charge, educate HCPs about a rare disease patient under their care, and potentially save lives.
FOUR FOUNDATIONAL PILLARS OF REAL-TIME ALERTS
WHAT IS THE PRODUCT LIFECYCLE?
The product lifecycle includes all the phases of a drug from research and development (which takes an average of 12 years) to launch as a branded medicine, as well as the seven years of exclusivity before having to come off patent.
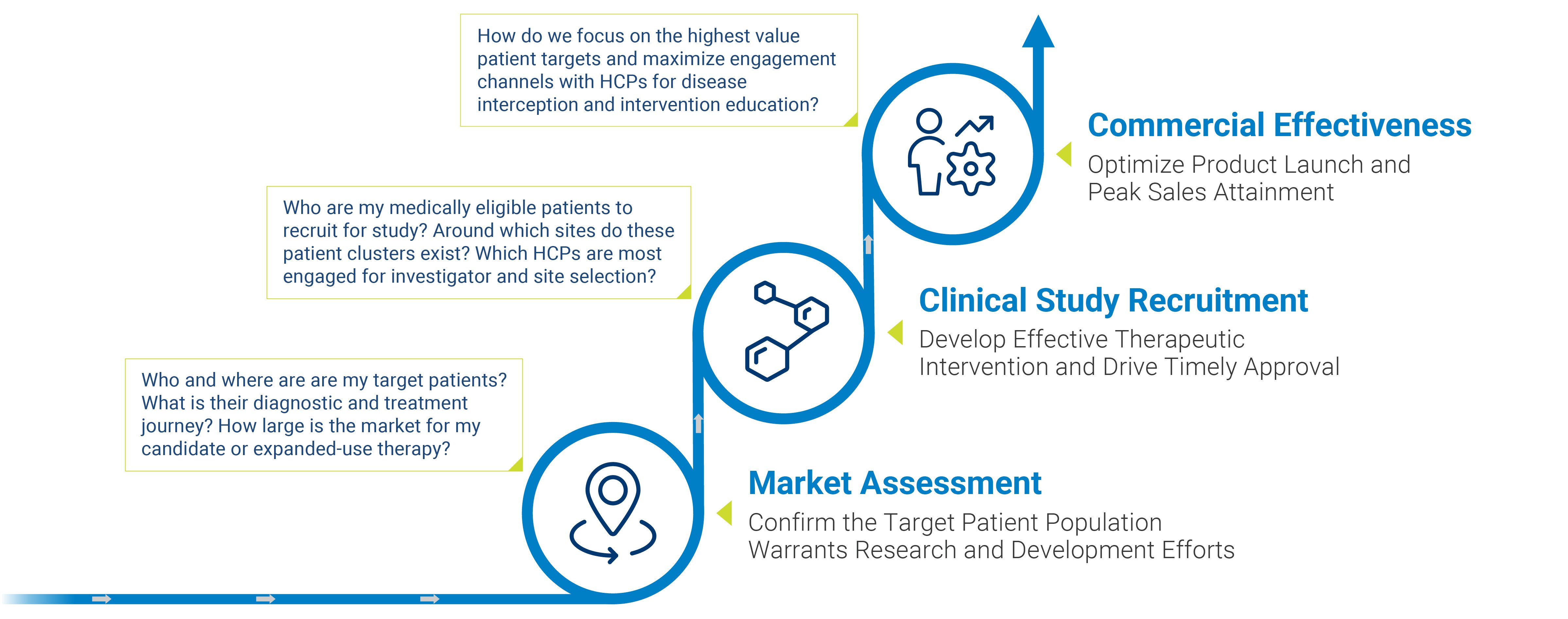
HOW IS ML/AI PATIENT FINDING BENEFICIAL DURING MARKET ASSESSMENT?
Market assessment is a crucial first step as it covers a drug’s potential reach, penetration, and lifetime value. Therapies designated for rare and specialty conditions necessitate assessment that goes far beyond combing through patient registries, mining clinical data, and interfacing with support groups. While this traditional approach to market evaluation might lead to an initial pool of diagnosed patients, the analysis is severely limited as those who are undiagnosed or misdiagnosed are typically excluded.
The rare disease patient population is significant as a group (nearly 30 million people) but individual specialty conditions generally affect no more than several hundred to a thousand patients, effectively making these disorders and the patients with them “invisible” to the healthcare system. Unlike common conditions, companies can’t expect these patients to come to them; patients must be uncovered to be converted.
Creating a Real Challenge for Life Science Companies
0%
of drug development efforts are abandoned due to the lack of uncovering a viable patient population
0%
of product launches miss their mark due to the lack of identification and understanding of the ideal patient
0%
of therapies in market fail to achieve peak sales due to the inadequate discovery of potential prescribers
To fully realize the ideal patient population for a new line of therapy, including those undergoing a diagnostic odyssey, ML/AI can uncover patients with a high propensity of disease affliction. In almost all cases, this increases a patient pool by 40x or more. Aside from enabling a better understanding of a disease’s epidemiology, symptomatology and prevalence rates, this technology maps the treatment journey. This allows companies to intercept patients based on key health events, sometimes even before they occur, using predictive analysis.
Although indications for rare and specialty diseases often lack competition, if a similar drug is in development and gets approved first, the patient pool can decrease significantly. In the assessment phase, drug companies can determine growth opportunities, including market size and unmet needs, the competitive landscape and competitor analysis. By identifying a more robust market opportunity including real patient population and potential demand, coupled with direct access to HCPs, clients can expect a more accurate forecast than is available through traditional market research. And access to better information allows better planning from the start.
HOW IS ML/AI PATIENT FINDING BENEFICIAL DURING CLINICAL TRIALS?
ML/AI can uncover clusters of undiagnosed and/or misdiagnosed patients during all clinical trial phases. Patients are also mapped by location, leading to individuals who are traditionally less likely to seek out specialty care (for example, those located in rural areas). As 85% of rare diseases are genetic, once a candidate is revealed, it’s likely that they may be part of a patient cluster, which can be a difference-maker for companies deciding where to set up a trial site.
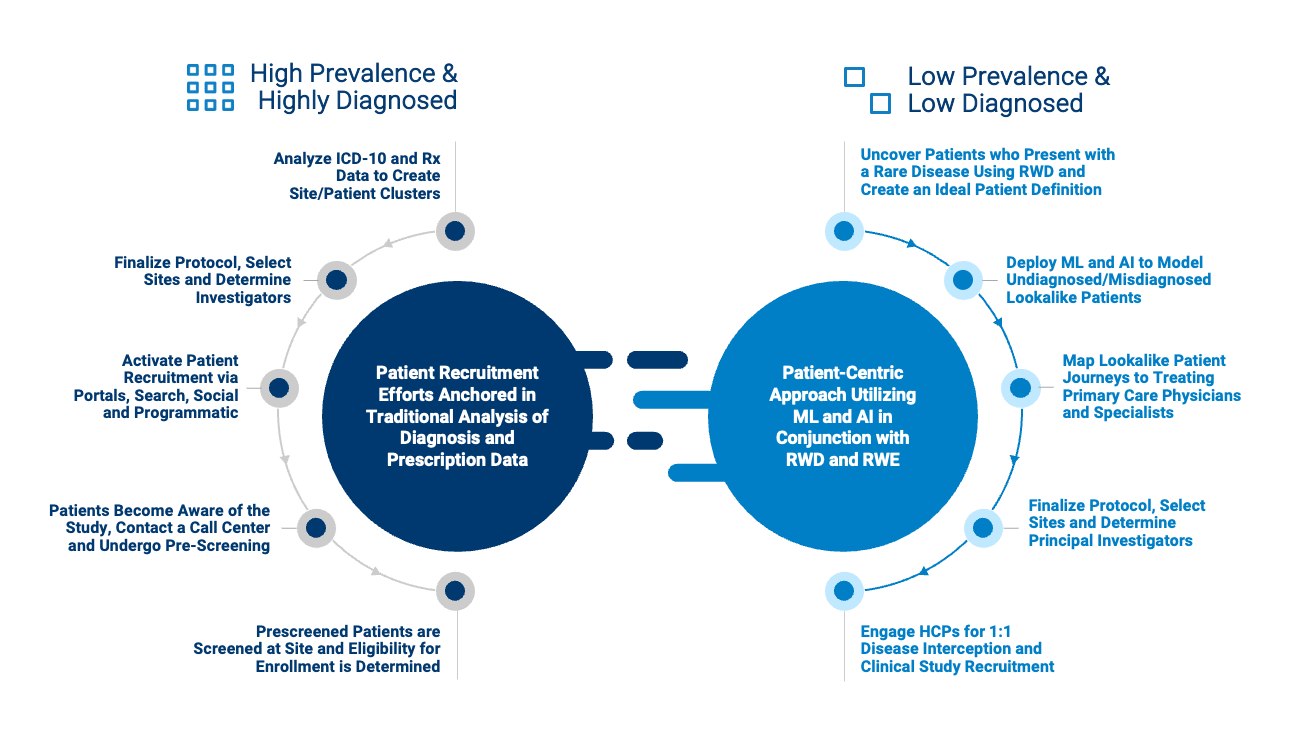
Again, instead of waiting for rare patients to appear, with ML/AI, companies can choose a location near highly likely or confirmed patients. If a trial site is convenient for patients, more individuals are likely to enroll and be retained. In addition to knowing where these anonymized patients reside, their treating and referring HCPs and can also be educated about the clinical study. This ability to intervene in diagnosis and provide access to a trial can completely alter patient outcomes.
Once it’s clear a viable patient pool exists, it can be refined further to match highly specific trial criteria, for instance, patients who have recently relapsed or have a certain comorbidity.
This technology can be utilized to minimize protocol revisions by verifying patients are medically eligible and thus have a low propensity for screening failure. Clients can also incorporate behavioral variables into their ideal patient definition to ensure the highest likelihood of enrollment and retention. The impact is significant, with a 35% reduction of the typical multi-year study recruitment timeline and millions of dollars saved in the process.
HOW IS ML/AI PATIENT FINDING BENEFICIAL FOR ACHIEVING PEAK REVENUE?
Establishing a therapy as the standard of care and thus setting up a trajectory towards peak revenue can be accomplished by discovering, mapping, and engaging ideal HCPs through ML/AI.
Uncovering patients through 1:1 engagement with influential physicians maximizes effectiveness and efficiency, as specialty and rare disease sufferers trust the opinions of their HCPs over messages via programmatic, social and television advertising. In fact, it’s common to add hundreds of new patient prescriptions within six months after utilizing ML/AI to uncover patients.
While campaigns can evolve once a product is in the field, there is no market without a viable patient pool – and even a single rare and specialty patient is hugely valuable. Because of this, precision medicine necessitates a precise approach to patient-finding. For healthcare and life sciences organizations concentrating on the specialty or rare disease market, the use of ML/AI is indispensable for making smarter decisions with a higher probability of success. Standardizing on this approach creates a healthier portfolio, provides the agility to capitalize on new opportunities and helps shape long-term innovation.
IPM.ai CREATES VALUE BY:
Improving Investment and Exit Options
through larger rounds of equity investment, better licensing terms, and improved acquistion outlook
Forecast Likely Demand
by linking the ideal patient population with treating HCPs, inferred specialists and Key Opinion Leaders/Influencers
Validating the Size of the Market
by uncovering undiagnosed or misdiagnosed patients with a high-probability of disease manifestation
WHAT ACTION CAN I TAKE NOW TO UNCOVER PATIENTS FOR MY DRUG?
By working with an accredited ML/AI insights-as-a-service (IaaS) company like IPM.ai, life science companies can uncover patients who would likely go undiagnosed otherwise. ML/AI should be the first option in patient-finding, enabling more people to be intercepted at a critical moment in their treatment journey and furthering better outcomes.
LET'S CONNECTLet's Connect
For more information, an initial consult, a career opportunity or media request, get in touch with us.